Moderna
@moderna_tx
Followers
146K
Following
542
Media
2K
Statuses
3K
Our mission is to deliver the greatest possible impact to people through mRNA medicines.
Cambridge, MA
Joined December 2013
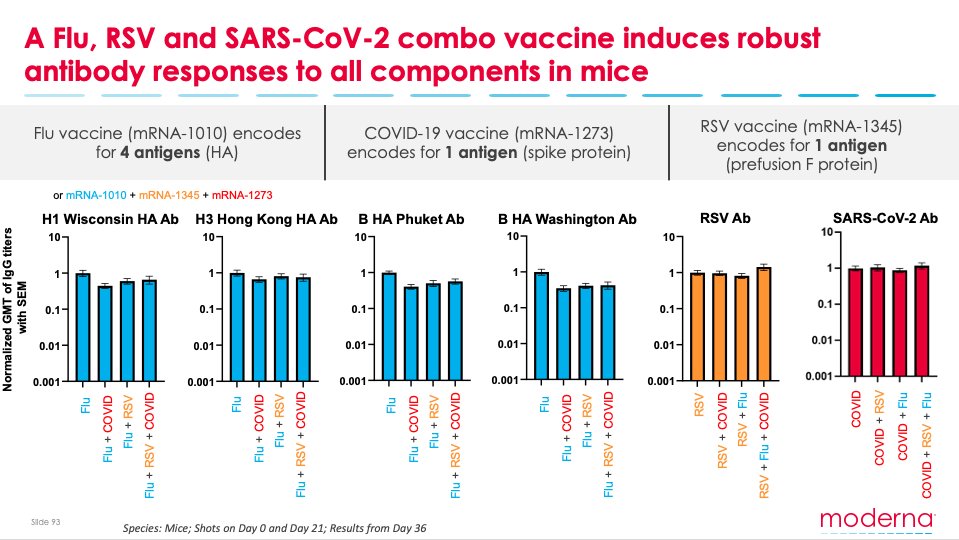
Today, we shared positive pre-clinical data demonstrating our ability to combine 6 mRNAs against 3 different respiratory viruses in 1 vaccine: COVID-19 booster + Flu booster + RSV booster. #mRNA
783
5K
16K
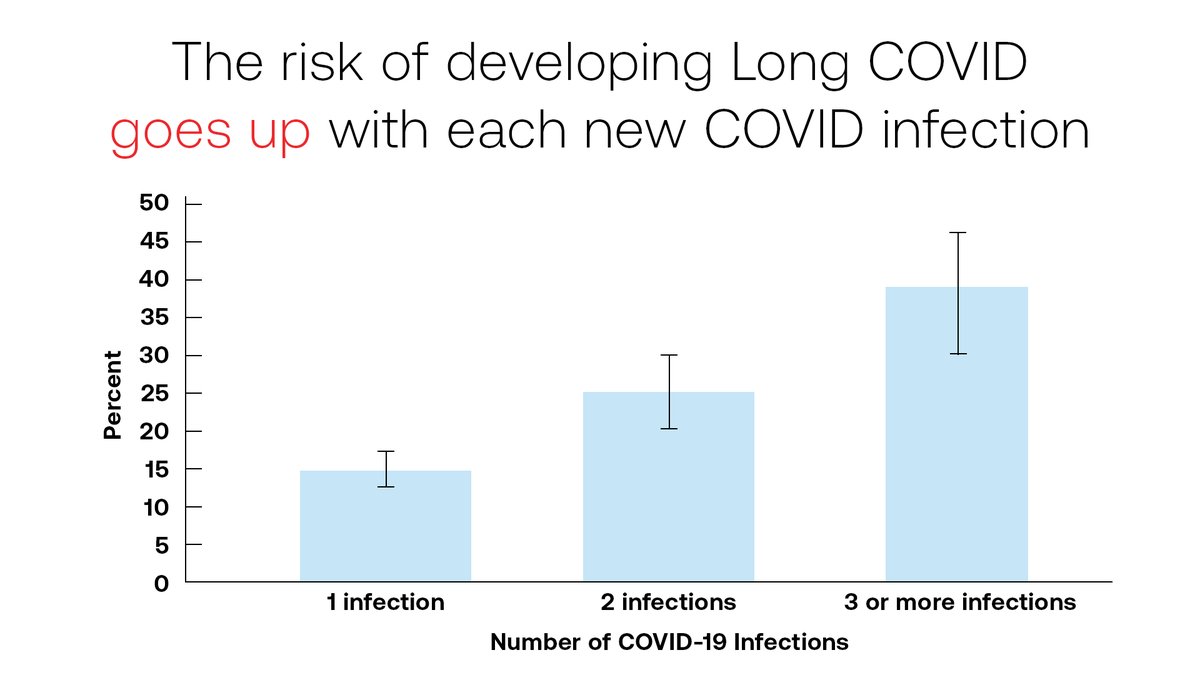
Your risk of #LongCOVID rises with each additional infection. A recent study has shown that by their third COVID infection, patients have a 40% chance of developing Long COVID symptoms. Learn more about the threat of Long COVID:
1
2K
3K
None of this would be possible without our partnerships, and we are grateful to @gatesfoundation, @scrippsresearch, @UTSA, @GWtweets, @fredhutch, and @EmoryUniversity.
12
104
986
We just confirmed that the @US_FDA VRBPAC recommended that the FDA grant an Emergency Use Authorization for our COVID-19 vaccine candidate, mRNA-1273. 20 VRBPAC members recommended for EUA, 0 members voted against, and 1 abstained. Read more:
36
312
1K
We just announced an expansion of our agreement with @BARDA for up to an additional $472 million to support our late stage clinical development of mRNA-1273, including the execution of a 30,000 participant Phase 3 study in the U.S. Read more:
52
351
897
We just announced that the first participant has been dosed in the NIH-led Phase 1 study of our #mRNA vaccine (mRNA-1273) against novel coronavirus
37
384
830
We're announcing positive interim data from the Phase 2/3 KidCOVE study of our #COVID19 vaccine (mRNA-1273) in children 6 months to under 2 years and 2 years to under 6 years of age.
1
313
782
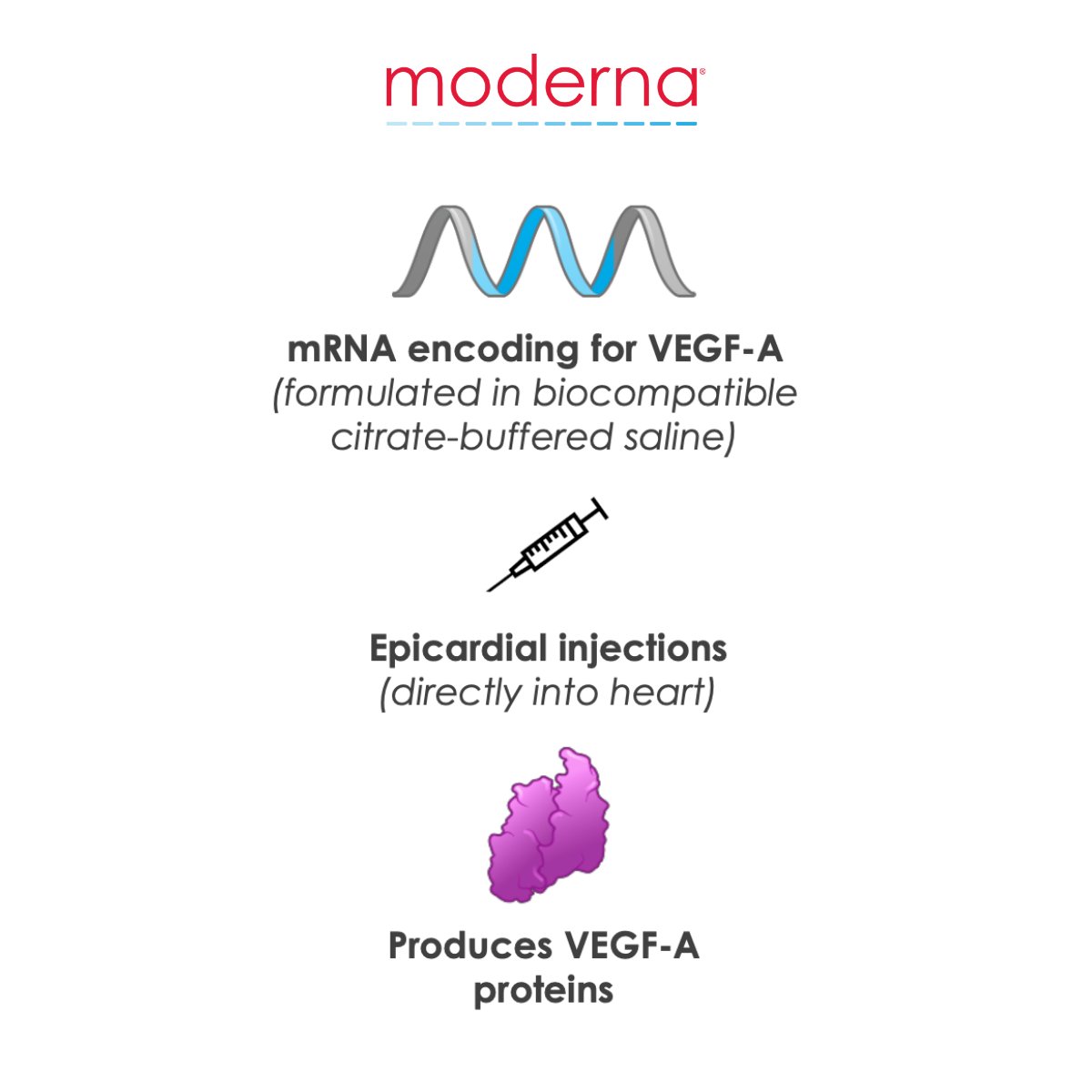
What if #mRNA could treat life-threatening #cardiovascular diseases such as #HeartFailure? We are collaborating with @AstraZeneca on an mRNA therapeutic (AZD8601) that encodes for VEGF-A to promote recovery of cardiac function through tissue regeneration.
18
451
820
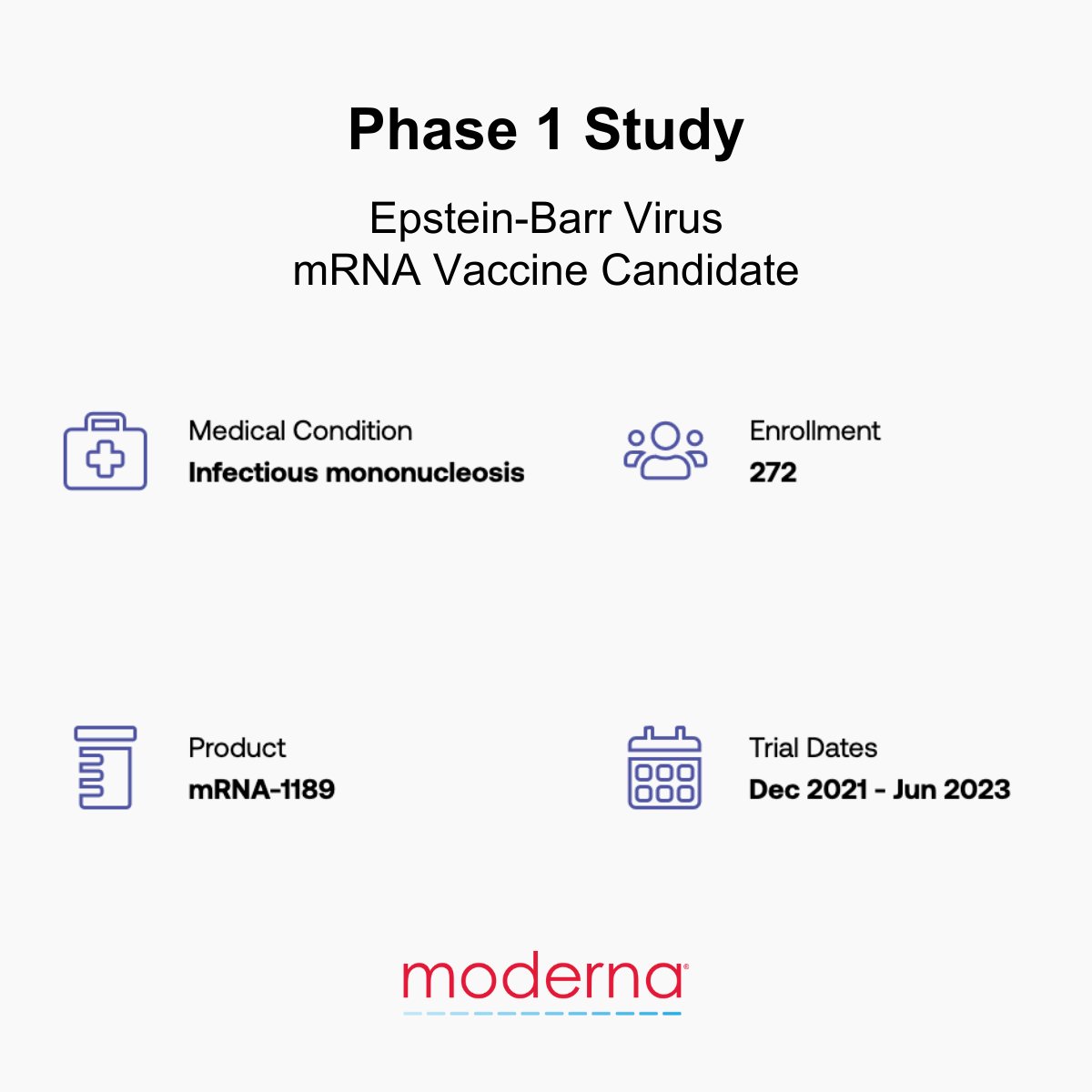
Moderna is developing an investigational mRNA vaccine against cytomegalovirus. Learn more about #CMV here: #StopCMV #CMVawareness
22
139
767
Long COVID is having a major impact on the productivity of health systems and the wider workforce. One recent estimate suggests that #LongCOVID may have cut labor supply in the European Union by more than 1 million full-time workers in 2022.
1
260
642
There are more adults ages 35-49 living with #LongCOVID than any other age group. Long COVID can impact everyone, regardless of age or health status. The only way to not get Long COVID is to not get COVID. Learn more about Long COVID:
0
282
607
Respiratory viruses are a leading cause of many hospitalizations and deaths. We believe Moderna could be first to market with a COVID + Flu + RSV booster vaccine. A single vaccine to cover multiple viruses. #IDWeek2021
36
115
520
We are pleased to announce that today we completed our submission to the @US_FDA, which we began on Wednesday, for the evaluation of a booster dose of the Moderna COVID-19 vaccine.
45
116
506
We are pleased to share that Health Canada has authorized our #mRNA COVID-19 vaccine in Canada to include immunization to prevent COVID-19 caused by the SARS-CoV-2 virus in adolescents 12 years of age and older under the Interim Order.
46
114
458
We just announced that the European Commission has approved an agreement to secure 80 million doses of mRNA-1273, with the option to increase their purchase to a total of up to 160 million doses. Read more: #mRNA
34
93
458
We are pleased to share a preprint of the preclinical manuscript for our #vaccine against #SARS-CoV-2 to potentially prevent #COVID19 disease. We thank the NIAID team for their collaboration with our infectious disease research team. @NIAIDNews.
17
187
437
We just announced that the first participants in each age cohort have been dosed in the Phase 2 study of our mRNA vaccine (mRNA-1273) against novel coronavirus. Read more: #mRNA
28
153
414
To provide additional transparency in context of pandemic, the protocol for the Phase 3 COVE study of mRNA-1273 being conducted in collaboration with the NIH and BARDA is now available: #mRNA
11
172
383
Earlier today we announced that we shipped our variant-specific vaccine candidate, mRNA-1273.351, to the @NIH for a Phase 1 clinical trial that will be led and funded by the NIH’s National Institute of Allergy and Infectious Diseases (NIAID). Read more:
8
130
360
We just announced an agreement in principle with the Australian Government to build a state-of-the-art #mRNA vaccine manufacturing facility in Victoria, Australia including access to our mRNA development engine. Read more:
22
56
354
This evening, we are highlighting the publication of antibody persistence data out to 6 months following the second dose of the Moderna COVID-19 Vaccine in @NEJM . Read more:
26
78
347
We just announced an amendment to the current supply agreement with the United Kingdom government for an additional 2 million doses of mRNA-1273, our #mRNA vaccine candidate against COVID-19. Read more:
31
72
353
Today, we announced that we will build a state-of-the-art #mRNA facility in Africa to manufacture up to 500 million doses per year. Since our founding in 2010, our mission has been to make a transformative impact on human lives through our medicines:
30
67
352