Charlene Liao
@charlene_liao
Followers
175
Following
726
Media
9
Statuses
277
Founder, President and CEO Immune-Onc Therapeutics, Inc.
Palo Alto, CA
Joined October 2017
The publication of our IO-202 Phase 1 data in Blood Neoplasia marks an important moment for @ImmuneOnc and for patients with HMA-naïve #CMML and monocytic #AML, who urgently need better options. We are grateful to the patients, investigators, care teams and staff for this work.
Phase 1 results for IO-202, our anti-LILRB4 antibody, are now in @Bloodneoplasia. The data show promising activity in #CMML and #AML, offering new hope for patients with limited treatment options. Read more: https://t.co/occ4adhbGw
0
0
0
This is an important step for @ImmuneOnc and for patients with #HCC. We look forward to learning how IO-108 may add to existing therapies and provide more choices for #patients. I’m very grateful to our team, our partners at Roche, and clinical investigators leading this effort.
The first patient has been dosed in our global Ph 1b/2 trial evaluating IO-108 for 1st-line treatment of HCC in clinical collaboration with Roche! A major step forward in our mission to advance cancer care. Learn more: https://t.co/eTXbgkMAns
2
0
8
Congratulations to Liqun Luo. We are proud of your accomplishments and happy for you.
The winner of the 2025 NAS Award in the Neurosciences is #NASmember Liqun Luo of @Stanford and @HHMINEWS for advancing understanding of the mechanisms of neural development, neuronal diversity, and brain wiring! Learn more: https://t.co/K1aRjrrOKo
#NASaward #neuroscience
0
7
19
Thank you to Dr. Gabe Mannis for presenting our impressive data of IO-202 for #CMML at #ASH2024. It was wonderful to meet you and additional investigators including Dr. @DanPollyea and our supporter @LLSusa CSO Dr Lee Greenberger!
We’re back from #ASH2024 and inspired by the energy and engagement around our oral presentation on IO-202 for #CMML. This is a key step toward new hope for people with this rare blood cancer. Thank you to everyone who joined us. ICYMI: https://t.co/qnMjahl8ac
0
0
2
I am inspired by the dedication of our team and grateful for the patients and clinical investigators who made this progress possible. These results bring us closer to transforming care for #CMML patients who urgently need innovative therapies.
Today, we are proud to present groundbreaking new Phase 1b data on IO-202 for #CMML at #ASH2024! ✔️ CR: 50% | ORR: 66.7% ✔️ High LILRB4 expression: CR: 83%; ORR: 100% ✔️ 40% bridged to transplant ✔️ Additional clinical benefits Learn more: https://t.co/qnMjahl8ac
0
0
2
Fantastic presentation of groundbreaking Phase 1b data on IO-202 for CMML at #ASH2024! ✔️ CR: 50% | ORR: 66.7% ✔️ High LILRB4 expression: CR: 83%; ORR: 100% ✔️ 40% bridged to transplant ✔️Additional clinical benefits
@ImmuneOnc will present important updates on its Phase 1b data on IO-202 for CMML. Don’t miss the oral presentation at #ASH2024 on Monday!
0
0
1
@ImmuneOnc will present important updates on its Phase 1b data on IO-202 for CMML. Don’t miss the oral presentation at #ASH2024 on Monday!
1
1
1
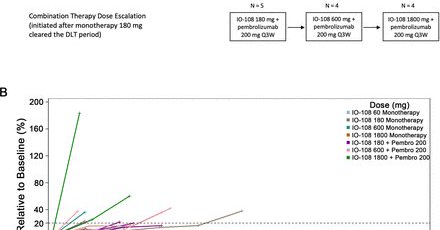
Our Phase 1 data on IO-108 was published in @jitcancer · Durable responses in advanced solid tumors · Promising monotherapy & combo results with pembrolizumab · Potential to overcome resistance to checkpoint inhibitors https://t.co/eHYBaPmr0F
New #JITC article: Phase I dose escalation study of IO-108, an anti-LILRB2 antibody, in patients with advanced solid tumors https://t.co/f3beNUtfAG
@ANaingMD
0
0
0
New #JITC article: Phase I dose escalation study of IO-108, an anti-LILRB2 antibody, in patients with advanced solid tumors https://t.co/f3beNUtfAG
@ANaingMD
1
2
5
@JohnCendpts this paper showcased one patient achieving a durable complete response (CR) for over two years. The CR was observed in a patient with Merkel cell carcinoma who progressed with prior treatments of pembrolizumab, followed by a combination of nivolumab and ipilimumab.
0
0
0
Our Phase 1 dose escalation data for IO-108, a first-in-class anti-LILRB2 antibody, recently published in @jitcancer, showed monotherapy and combination efficacy in advanced solid tumors.
jitc.bmj.com
Purpose In this first-in-human dose escalation study, the safety and efficacy of IO-108, a fully human monoclonal antibody targeting leukocyte immunoglobulin-like receptor B2 (LILRB2), was investig...
Our Ph 1 data on IO-108 was published in @jitcancer ! · Durable responses in advanced solid tumors · Promising monotherapy & combo results with pembrolizumab · Potential to overcome resistance to checkpoint inhibitors Read the full press release: https://t.co/bScmePooLs
1
0
3
The induction of these anti–IFN-α autoantibodies was temporally linked to a decline in IFN-α itself, suggesting that elicitation of these autoantibodies may be a mechanism to temper inflammation in the respiratory mucosa.
Autoantibodies to interferon-α (IFN-α) in the blood are associated with severe acute COVID infection. A new study shows that transient IgA1 autoantibodies to IFN-α in the nose were associated with milder cases of COVID.
0
1
2
Congratulations to all! Special thanks to #patients who participated in this study.
0
0
1
I’m proud to share that @ImmuneOnc's new data on IO-202 in chronic myelomonocytic leukemia (CMML) will be featured in an oral presentation at #ASH24! This is a significant moment in our mission to transform outcomes for CMML patients who have limited treatment options.
We're thrilled to announce that Immune-Onc will present new IO-202 data for chronic myelomonocytic leukemia (CMML) at #ASH24! Join us to learn about the potential of our anti-LILRB4 antibody in addressing CMML, an underserved cancer with limited treatments https://t.co/vWEmFZyDof
2
1
4
Check this out from my good friend Dr Ruth Ann Crystal. She provides an informative and important advice. I also like the monoclonal antibody that prevents RSV infection, with its long half-life and long-lasting effects.
I am proud to partner with @Sanofi and contribute to @ContempOBGYN! Check out my article on infant respiratory syncytial virus (#RSV) disease prevention, including tips to help support healthy outcomes for our tiniest patients: https://t.co/uj9rt655tQ
#SanofiSponsored
0
0
1
Thank you @JohnCendpts for writing about your journey as a cancer patient and for shedding light on Merkel cell carcinoma (MCC) and the perseverance of drug developers to get 3 new medicines approved that mobilize our immune system to fight this rare cancer. We have more to come!
Trending again today, likely because it was featured in the weekend report. If you didn't see it, or promised yourself you'd read it later, I'm offering it back up. It's a bit far-fetched, but we have to do something about rare disease research to make it more feasible.
0
0
2
I’m pleased to announce positive new data from our Phase 1b trial of IO-202 in #CMML. We look forward to sharing these data with the medical community at #EHA2024 and working with the FDA to discuss plans for a registrational study to bring this innovative medicine to patients.
New from @ImmuneOnc at #EHA2024! Today, we will present additional positive interim Ph 1b expansion data for IO-202 in #CMML patients. The study shows early and sustained complete remissions among patients, regardless of their prognosis or mutation status. https://t.co/l7ggmOUyk8
0
0
8
I’m excited to announce that @ImmuneOnc will present new data on IO-202 in #CMML at the #EHA2024 conference in Spain next month. If you plan to attend #EHA2024, please get in touch to say “Hello” and learn more about our work in blood cancer. I look forward to seeing you there!
#News: We look forward to presenting interim clinical data from our Phase 1b trial of IO-202 for the treatment of chronic myelomonocytic leukemia (#CMML) at the upcoming #EHA2024 Congress. View the press release for details: https://t.co/shN9syGVJq
0
0
1
RETWEET if you believe! 🌟🔁 Tag two people or donate $20 today! This #MyelomaAwarenessMonth and year-round, your donations fuel our mission. ✨ Donate now 👉 https://t.co/wOmMR8HNiz
1
13
36
Another great recognition for @ImmuneOnc ! I am immensely proud of our team for their commitment to making a difference in the lives of patients and I am grateful to the @SFBTMultimedia for shining a spotlight on the vital role of women leaders in the life sciences industry.
Exciting News! The @SFBusinessTimes recognized our Orphan Drug Designation for IO-202 for #CMML as a recent breakthrough by women-led life sciences companies in the Bay Area. Read more here: https://t.co/p2MQ0zvnlf
0
0
5