MDS Hub
@MDS_Hub
Followers
2K
Following
570
Statuses
1K
Lifting levels of knowledge in myelodysplastic syndromes (#MDS). A global online platform providing latest treatment options and medical information. #mdssm
Joined November 2020
A big thank you to our funders, who make it possible for us to deliver the MDS Hub Bristol Myers Squibb ThermoFisher Scientific Astex All content is developed independently by SES in collaboration with an expert steering committee; funders are allowed no direct influence on the content of the hub.
0
0
6
🎬 Discover the latest insights on MDS classification systems! Join Pierre Fenaux as he delves into his updated approach in our new video. Don't miss this pivotal discussion from our MDS Hub Steering Committee meeting. 👉 #MDS #MedicalScience
0
0
3
CONGRESS | #ASH23 Uwe Platzbecker @UwePlatzbecker @UniLeipzig presents from IMerge ph3 trial of imetelstat vs placebo in patients with non-del(5q) LR-#MDS R/R or ineligible for ESAs. ▪️Of 33 patients who achieved ≥24-wk RBC-TI with imetelstat, 63.6% maintained for ≥1-yr; majority had normal karyotype (57%), prior ESA treatment (90%) and were RS+ (71%) ▪️Median duration of RBC-TI in 1-yr TI responders (imetelstat arm) =123 wks, with Hb increase during the longest TI interval = 5.2g/dL ▪️No patients progressed to AML ▪️Of the 21 imetelstat-treated ≥1-yr TI responders, 7 had cytogentic abnormality at baseline, and 4 had a complete cytogenetic response ▪️Gr3-4 thrombocytopenia (67%) and neutropenia (95%) were common AEs in ≥1-yr TI responders @ASH_hematology #MDSsm #leusm
0
2
9
RT @AML_Hub: CONGRESS | #ASH23 Enrico Attardi @enrico_attardi @unitorvergata @StJude dissects ICC “diagnostic qualifiers”, using a cohort o…
0
4
0
CONGRESS | #ASH23
@Santin13Valeria @UNI_FIRENZE Imetelstat was superior to placebo in inducing ≥8wk & ≥24 week RBC-TI, regardless of number of baseline mutations ▪️Improved RBC-TI response to imetelstat vs placebo particularly noted in patients with SF3B1 mutations (≥8wk RBC-TI 48.8% vs 16.3, p=0.001) ▪️Patients carrying spliceosome mutations had significantly improved response to imetelstat than placebo (≥8-wk RBC-TI 43.8% vs 14.9%, p=0.001) ▪️Trend towards higher RBC-TI response rate with imetelstat vs placebo in patients with poor-prognosis genes (ASXL1, TP53, ETV6, RUNX1, EZH2): 8 weeks, 31.6% vs 9%; 24 weeks, 9.1% vs 0%) @ASH_hematology #MDS #MDSsm #leusm
0
9
17
CONGRESS | #ASH23 Esther Natalie Oliva @GOM_rc presents an analysis of patient-reported outcomes in patients in the COMMANDS trial. ▪️Good side effect tolerability in both luspatercept and epoetin alfa arms (FACT-An item GP5) ▪️Improved times to sustained improvement to 24 weeks in luspatercept vs epoetin alfa arm for 8 EORTC QLQ-c30 domains, including role functioning (p=0.034), cognitive functioning (p=0.002) and pain (p=0.005), dyspnea (p=0.035), insomnia (p=0.045), appetite loss (p=0.001), constipation (p=0.001), and financial difficulties (p=0.006) ▪️FACT-An time to sustained improvement was similar between arms @ASH_hematology #MDS #MDSsm #leusm
0
0
3
CONGRESS | #ASH23 Valeria Santini @Santin13Valeria @UNI_FIRENZE presents long-term 26 month follow-up of patients with LR #MDS intolerant/ineligible for ESAs in the MEDALIST trial. ▪️Uninterrupted RBC-TI ≥1 yr was achieved by 41.3% of patients who achieved RBC-TI ≥8 wks, and 64.6% who achieved RBC-TI ≥16 wks ▪️Long-term safety was consistent with short-term, with TEAES similar between luspatercept and placebo arms when exposure-adjusted; fatigue rates decreased with increased luspatercept dose and increased Hb levels @ASH_hematology #MDSsm #leusm
0
5
17
CONGRESS | #ASH23 Rami Komrokji @Ramikomrokji @MoffittNews presents a mutational burden analysis from the COMMANDS trial. ▪️Luspatercept showed broad activity across MDS-associated mutations, including difficult-to-treat ASXL1 ▪️Response rates increased with luspatercept vs epoetin alfa in patients with 1–3 mutations overall and in RS+ subgroup; in RS- subgroup, luspatercept and epoetin alfa similar despite mutational burden differences ▪️Patients who were RS- in the epoetin alfa arm had lower mutational burden at baseline compared to RS+ (p=000024) ▪️Benefit of luspatercept seen across IPSS-M risk levels @ASH_hematology #MDS #MDSsm #leusm
0
3
10
Elisabetta Sauta @ILeukemia summarizes the significant contribution of transcriptomic features (40%), plus somatic gene mutations and cytogenetic lesions (24%), demographics (20%) and clinical features (15%) to survival prediction, in an integrative analysis of 389 patients with #MDS. By comparing to IPSS-M, she demonstrates enhanced clinical outcome prediction. @genomed4all @SYNTHEMA_EU @ASH_hematology #mdssm
0
1
6
RT @AML_Hub: CONGRESS | #ASH23 Caspian Oliai @UCLAJCCC @UCLAHealth discusses ph1b trial of Orca-T. 1-yr RFS was similar between patients ag…
0
2
0
CONGRESS #ASH23 | Moshe Mittelman presented results from MATTERHORN, an ongoing, double-blind, Phase III trial of roxadustat for treatment of anemia in pts with low-risk #MDS. At week 28 more pts in the roxadustat arm compared with the placebo arm were TI responders (47.5% vs. 33.3%), but this was not statistically significant. However, roxadustat was well-tolerated. #MDSsm
0
2
4
CONGRESS #ASH23 | Rami S. Komrokji discussed results from the Imerge phase III study evaluating the efficacy of imetelstat across different IPSS risk categories. The TI rate for all IPSS risk groups at ≥1-year were 12.5% vs 2.6% for imetelstat vs placebo in the low risk pts and 15.8% vs 0% in intermediate risk pts. #MDS #MDSsm
0
9
24
CONGRESS #ASH23 | Guillermo Garcia-Manero presented results from the phase III COMMANDS trial of Luspatercept Versus Epoetin Alfa in ESA-Naive pts with low-risk #MDS. 2.7% and 3.3% of pts in the luspatercept and epoetin alfa arms, respectively, progressed to #AML. With luspatercept, RBC-TI duration and erythroid responses were superior compared with epoetin alfa. #MDSsm
1
8
17
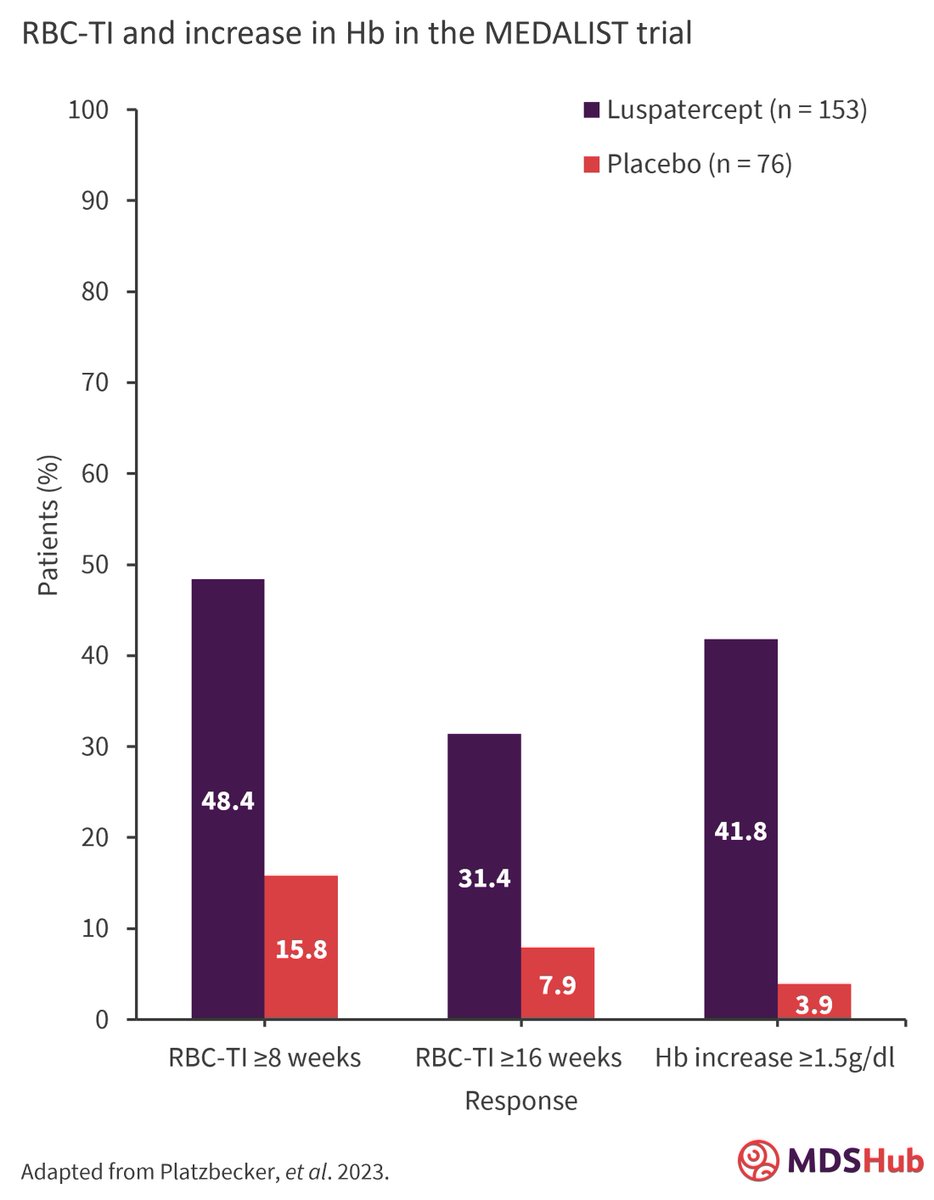
What is the long-term benefit of luspatercept in the treatment of anemia for transfusion-dependent patients with ring sideroblast-positive, lower-risk myelodysplastic syndromes who are erythropoietin-stimulating agent refractory/intolerant? Read our summary of a long-term analysis from the phase III MEDALIST trial 👇 #MDS #leusm
0
0
1