Exon 20 Group, a multi-stakeholder organization
@Exon20Group
Followers
2K
Following
2K
Statuses
1K
Global org: patients/carers, HCPs, pharma, labs, scientists, working to cure #NSCLC #HER2 & #EGFR #Exon20 insertions (in 25 cancers +) [email protected]
82 countries served
Joined July 2017
Don’t let your #EGFR #Exon20 and #HER2 #Exon20 patients miss out on the wonderful, caring community of patients and care partners (+ family members) in the multi-stakeholder @Exon20Group We have peer-to-peer counseling through our Angel Buddies program, oncology nursing services, weekly virtual meetings, and direct patient navigation plus 20 closed Facebook groups and Inspire. Ami and mobo as well as the drugs in the clinical trials pipeline are vitally important & manageable. We are looking for additional investigators, so let us know! Clinicians, molecular profiling labs welcome. Join our Exon 20 International Research Consortium! All services are free, & marcia@exon20group.org
0
3
20
RT @maraantonoff: The honor of this opportunity does not escape me. I am sitting on the shoulders of the 15 inspiring @WomenInThoracic Pres…
0
17
0
RT @StephenVLiu: Dr. Pasi Janne at #ITCD2025 discusses #EGFR TKI resistance and how we can overcome, or, more importantly, prevent resistan…
0
11
0
It was very sad day for the Exon 20 Group and our EGFR Exon 20 Warriors when the decision was made to withdraw mobocertinib from the market. While we are delighted and grateful to have a first-line therapy in PAPILLON’s amivantamab + chemo, doubling the PFS vs. chemo alone, we still mourn the loss of the September 15, 2021 accelerated approval for mobo in second-line where we saw one, two, three-plus years PFS in some patients. When companies are faced with such a challenge based on an unexpectedly disappointing Phase 3, the way to respond is the way @TakedaOncology did—with class and integrity, with a compassionate use program for patients to continue on mobocertinib, and with a commitment to supply every patient still on the drug after the final withdrawal date until disease progression. Takeda and its Here2Assist program went to great lengths to reassure patients and be responsive to HCPs.
0
4
12
Absolutely spot on, and every company needs to. We miss the years when 5 companies had expanded access programs, and several EAPs reached more than 80 countries.
@Exon20Group @TaihoOncology Great news! — Hoping now @TaihoOncology will actually consider implementing an expanded access/compassionate use program for Zipalertinib to address the unmet need for options other than ami for egfr exon 20 patients so they can “improve the lives of patients with cancer…”.
0
1
1
Huge and continuing promising news about zipalertinib (formerly CLN-081/TAS-6417) which is no surprise to the Exon 20 Group and our EGFR Exon 20 Warriors. Congrats to @TaihoOncology and Cullinan Therapeutics and all the patients, care partners, investigators, and study teams involved in the Phase 2b for zipalertinib.
1
2
7
RT @cure_today: How Subcutaneous vs IV May Affect Treatment Adherence in Cancer Care @lungoncdoc @MDAndersonNews #LungCancer #Oncology http…
0
5
0
RT @lungoncdoc: ‼️Phase 2 SKIPPirr: Reducing IRRs w/ IV amivantamab in EGFR+ mNSCLC post osimertinib/chemo ▫️Oral dexamethasone 8mg BID (D…
0
34
0
RT @DRCamidge: For a full thread on how MET amp more resembles a PDL1 type predictive biomarker and a summary of our decade of research - c…
0
3
0
RT @StephenVLiu: Report on NGS in squamous NSCLC @LungCaJournal from Dr. @Joshua_Reuss et al. Detected actionable genomic alterations in 22…
0
31
0
RT @jsoriamd: Cancer death rates in the U.S. have dropped 34% since 1991, averting over 4.5M deaths. However incidence continues to increas…
0
28
0
RT @HHorinouchi: 🔥@OncLive🆙 ✅Real World Evidence of Treatment Practices and Therapeutic Outcomes for Newly Diagnosed NSCLC Patients With No…
0
12
0
RT @LeXiuning: 📖Cancer statistics, 2025. 👉For the first time, the number of newly diagnosed female lung cancer cases has surpassed that of…
0
70
0
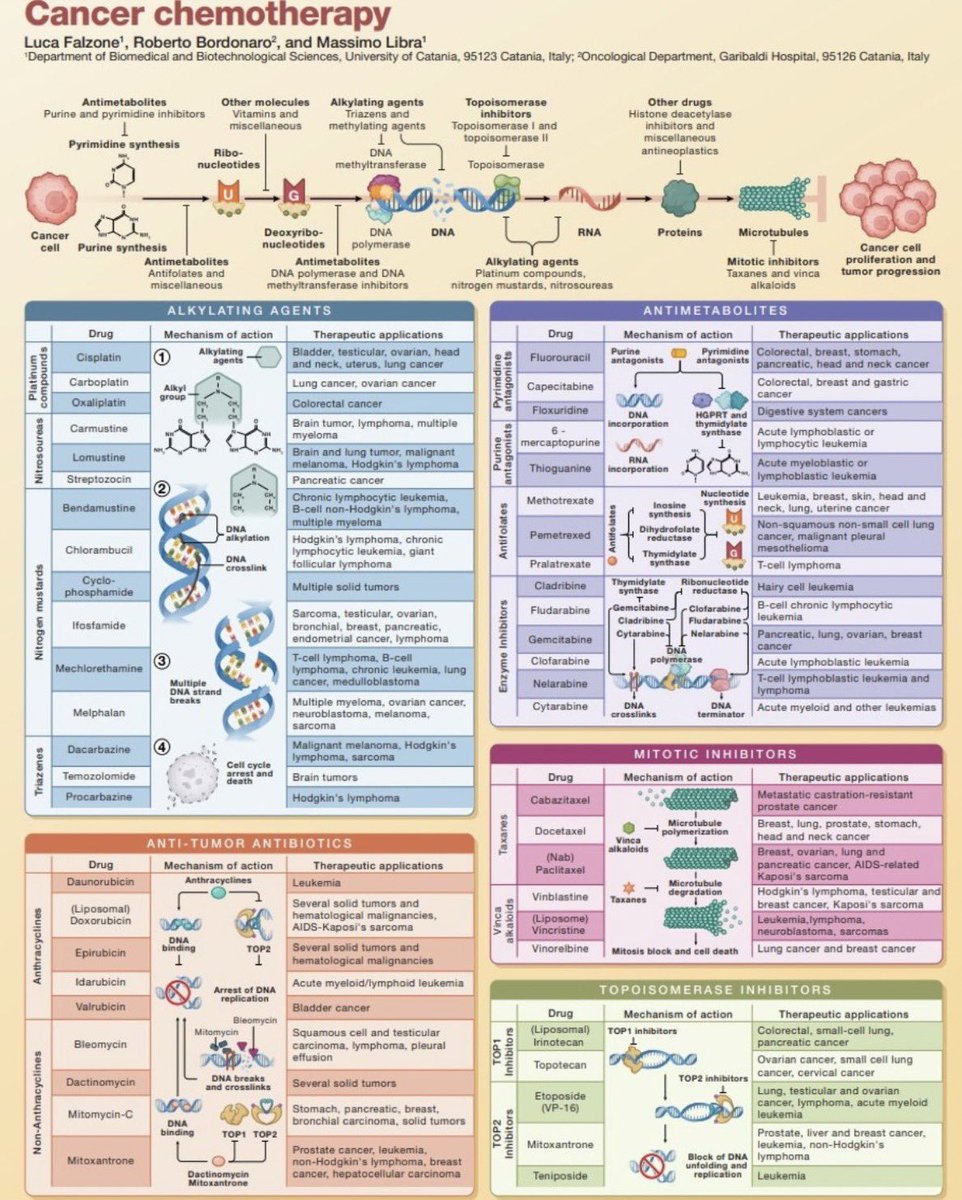
RT @SciencNews: Cancer Chemotherapy #meded #medx #chemotherapy #cancer #oncology #TICMED25 @oncodaily @OncoAlert
0
170
0
RT @RareCancers: We’re explaining the A to Z of words you might be hearing along your cancer journey. Today’s word is BIOPSY. A biopsy is w…
0
1
0
RT @oncodaily: @Latinamd @sanbenito @ASCO @DevikaDasMD @GlopesMd @AnaVManana We're delighted to inform you that your post has been publishe…
0
1
0
RT @lcfamerica: Radon testing is easy! Learn how to test for radon in just 3 simple steps. Awareness is key in lung cancer prevention. Big…
0
3
0
RT @StephenVLiu: Fortunately, lung cancer mortality is on the decline - but still so far to go. And unfortunately, significant disparities…
0
4
0
RT @StephenVLiu: This will be our biggest & best meeting yet! Register now and join us at the historic Austin City Limits for an unforgetta…
0
4
0
Crazy. Those pharma and biotech dollars are far better spent funding the Patient Services of advocacy organizations that molecularly match patients to clinical trials. It’s disheartening to work with companies (no matter what tumor type) where their CROs are callously invisible on the mission of patient recruitment for important clinical trials.
$BGNE $ONC on CRO cost/patient in onco, an reason to integrate clinical trial operations in-house CRO oncology trial cost-per-patient increased from ~$100K to ~$250-300K "based on anecdotal interviews with peer companies."
0
0
0