Christina Yap
@ChristinaBYap
Followers
828
Following
898
Media
38
Statuses
359
Professor of Clinical Trials Biostatistics. Group Leader Early Phase & Adaptive Trials. Interested in efficient trial designs & analysis @ICR_CTSU @ICR_London
England, United Kingdom
Joined November 2015
1/ NEW early phase dose-finding reporting guidelines for trial protocols (SPIRIT-DEFINE) and trial reports (CONSORT-DEFINE) to enhance transparency and reproducibility. @bmj_latest SPIRIT-DEFINE: https://t.co/ElyQ94G7jC CONSORT-DEFINE:
bmj.com
The CONSORT (CONsolidated Standards Of Reporting Trials) 2010 statement is the standard guideline for reporting completed randomised trials. The CONSORT Dose-finding Extension (DEFINE) extends the...
1
26
50
Congratulations to Cross-Sector Experience Award recipient @ChristinaBYap 🎉 Through this award, Prof Yap will work with AstraZeneca and Bayer to improve the design of early-phase trials, ensuring they are more effective and impactful for patients. 👉 https://t.co/40sFBbcHYp
1
1
11
A new insightful commentary from @haiyan_zheng, @ChristinaBYap and @JMSWason at @MRCNIHRTMRP is now in @BMCMedicine. Read more for insights on the interpretation of biomarker-guided clinical trials and appropriate use of terminology here: https://t.co/VMqLihSBMf
bmcmedicine.biomedcentral.com
0
3
5
Thank you for the kind invitation to share insights on the value of PROs in phase I dose-finding trials. A fantastic conference in the beautiful city of Barcelona - I’ve learnt so much too! Such a privilege to be part of it!
🩺 Phase 1 oncology trials focus on safety—but PROs can add real value. They complement clinician-reported dose-limiting toxicity (DLTs), offering a fuller view of treatment impact. Interest is growing across patients, researchers & regulators. @ChristinaBYap @EORTC #QoLConf25
1
1
8
Passionate about cancer trials, patient-reported outcomes (PROs) and innovative methods? Join the Early Phase and Adaptive Trials Group at @ICR_CTSU for an exciting undergraduate summer scholarship in early phase trials and PROs! Deadline: 26/01/25 https://t.co/L8c3urRBkJ
0
2
8
How about a two sequence (AB/BA) randomised blinded crossover design? PROs: Smaller sample size to achieve the same statistical power. Participants have the opportunity to try both durians & state their individual choice. (Well, I would love to be a participant of such a study!)
Discussing the optimal sample size needed to taste the difference between and high-end and standard durian. Should we be blinded? Any views on the design? #ESMOAsia24 @myESMO
0
1
6
Thank you for the kind invitation to speak at this Trial Methodology Showcase. I enjoyed discussing how innovative methods from cancer trials can inform approaches in other fields, including mental health, and vice versa. Looking forward to exciting synergies ahead!
@SGDPCentreKCL @richardaemsley We then kicked off with our first keynote speaker Professor Christina Yap @ChristinaBYap with her brilliant talk "Innovating Early Phase Trials: Advanced Methodologies for Enhanced Decision-Making"
0
3
13
Juanita Lopez, @ChristinaBYap & Richard Mair received an Experimental Medicine Award to build a next generation, glioma modular platform to evaluate the safety & efficacy of genotype matched investigational targeted therapies for malignant #BrainTumours
https://t.co/4EDEB9CMu3
0
3
15
Excited to have co-designed the efficient 5G platform trial, where we are testing several targeted treatments to help improve the lives of brain cancer patients. Grateful to our funders @CRUKresearch and @minderoo for making this trial possible! @ICR_DDU_IIT @ICR_CTSU @CRUK_CI
This exciting UK-wide clinical trial has the potential to improve outcomes for brain cancer patients by testing multiple medicines as treatments for people living with glioma. Congratulations to Juanita Lopez, @ChristinaBYap & Richard Mair for the award. https://t.co/RB6R41cvMB
0
3
30
0
0
1
2/ Honoured to collaborate with an outstanding multidisciplinary group, including @MLE_Alger
@EthanBasch1, @AlastairGreyst2, @BellKingKal, @Anna_Minchom, @LynneWagnerPhD and many others. A heartfelt thank you to my amazing joint hosts: Devin Peipert, @lee_1881 and @drmelcalvert.
1
0
1
1/ This paper highlights opportunities & challenges of integrating patient-reported outcomes in early phase trials, based on insights from an expert panel of academics, patient advocates, regulators & industry professionals, along with key recommendations. https://t.co/d3tbvJ0eCR
1
0
8
When you are coming down from the #ICTMC2024 conference and need some more trials methods research highlights then why not download the introductory @MRCTMRPDTP led podcast. First full episode drops Tuesday 8th October
podcasts.apple.com
Education Podcast · What on earth is Trials Methodology and why should we care!? These are just a few (!) of the questions we had when we became PhD students on the Trials Methodology Research...
0
13
21
We had a great start to #ICTMC2024 this week, with Trial Manager Morgaine Stiles presenting our demographics data collection project DISTINCT, and PhD student Georgiana Synesi, @GSynesi, presenting her work on inclusivity of our bladder cancer and head and neck cancer trials.
0
3
14
Yesterday at #ICTMC2024, principal statistician Jan presented extensions to the SPIRIT and CONSORT guidelines for early phase dose-finding trials (DEFINE).
1
2
13
Inspired to get creative with my poster for @ictmc2024 this week! I shared my work on bayesian generalised linear mixed models to optimise treatment dosage in early phase trials using Patient-reported Outcomes. #ICTMC2024 #clinicaltrials
0
0
20
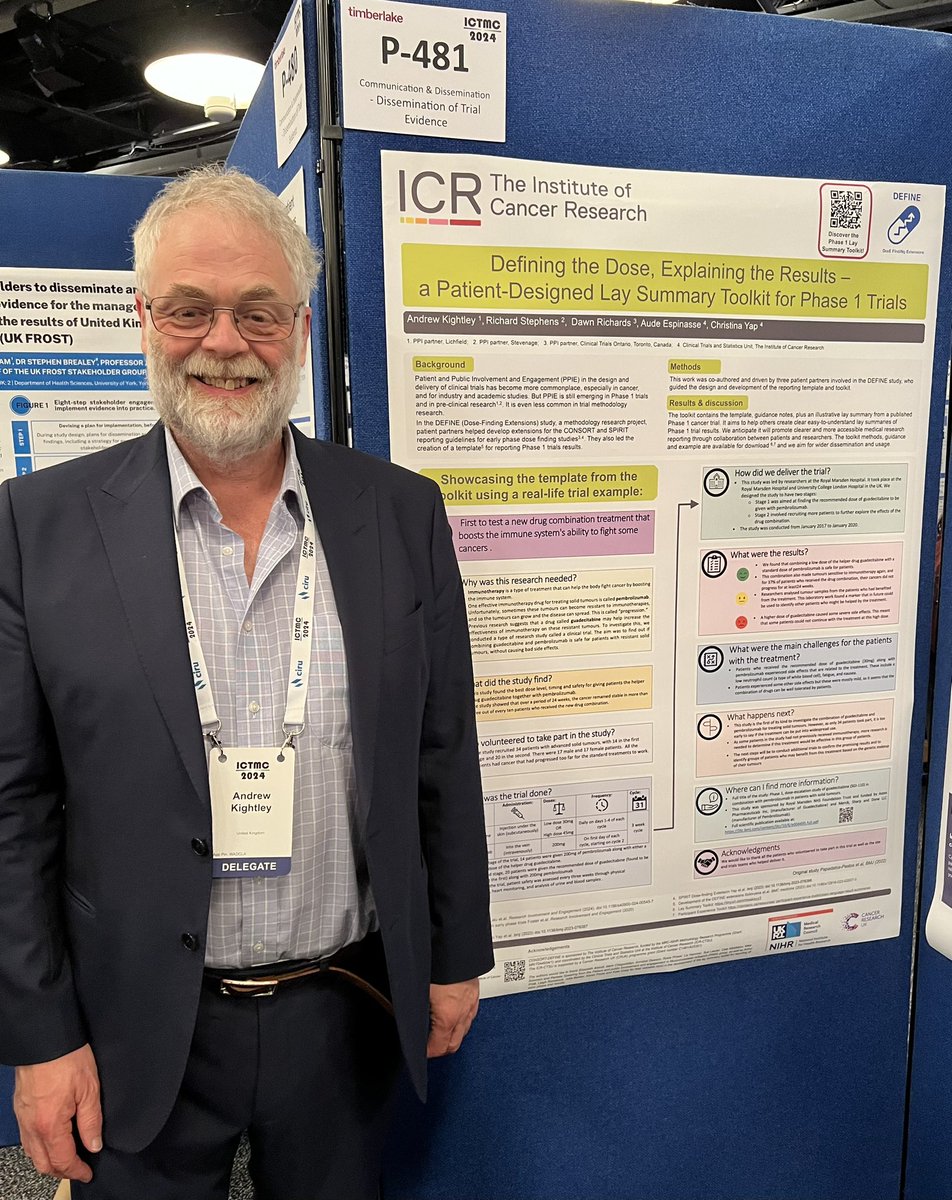
It’s been such a great delight to work with @ajmk, Richard Stevens, @TO_dpr and the team, on a toolkit to improve how early phase trial results are communicated to patients and the public. Proud of what we’ve achieved together. https://t.co/smarRq72Ul
Great to be presenting the Lay Summary Toolkit for early phase dose finding trials at #ICTMC2024 today. Thanks to all who worked on it @ChristinaBYap @ICR_CTSU, Richard Stephens, Aude Espinasse, @TO_dpr and many others.
1
1
14
Superb Congratulations, @haiyan_zheng ! This achievement is so well deserved. I’m really excited to see the valuable methodology contributions you’ll bring to cancer research, and I’m looking forward to continuing our work together on this important journey. 😊
I am deeply honoured to receive this prestigious #CRUK fellowship award with my STEEP project! Making precision medicine clinical trials more efficient is a passion of mine. I look forward to contributing more in the next 6 years! 📚📊🎯
1
0
5
📢#CancerTherapy claiming #TumourAgnostic potential❓ ✔ ORR≥20% in 2/3 of tumour types investigated (≥4) with ≥5 evaluable patients per tumour type 🔓@myESMO Tumour-Agnostic Classifier & Screener (ETAC-S) @Annals_Oncology
@BenWestphalen @VivekSubbiah @GPentheroudakis et al
ESMO announces the publication of the ESMO Tumour-Agnostic Classifier and Screener (ETAC-S), a new framework to assess the tumour-agnostic potential of #CancerTherapies with the aim to optimise the drug development process @Annals_Oncology. 🔗 https://t.co/8gmF94K9aq
1
7
21
Delighted to be involved in this new article by the Methodology for the Development of Innovative Cancer Therapies Taskforce (MDICT). Have a look: https://t.co/qrpE6r6W1g
MDICT 2024 recommendations for neoadjuvant early clinical trials now published. Led by ESMO-TAT chairs @lillian_siu @ElenaGarralda @AnastasiosStat2 and MDICT’24 team supported by @myESMO
https://t.co/RZdCpG0iWG
1
0
3
Exciting job opportunity for a talented statistician to join us at the Early Phase & Adaptive Trials Group @ICR_CTSU as a Trials Methodologist! If you enjoy developing and implementing efficient trial methods to impact patients’ lives, come and join us!
0
10
20